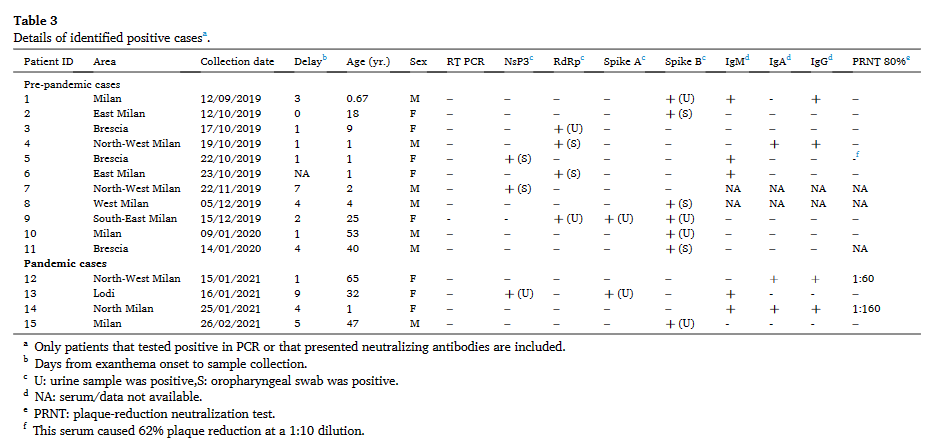
Independent confirmation of the Italian findings of SARS-CoV-2 RNA within samples dating as early as September 2019
When not just one but two studies across the same time period independently verified the circulation of SARS-CoV-2 within the prepandemic period in Italy.....
While there are enough negative controls and positive samples within the study https://www.sciencedirect.com/science/article/pii/S0013935122013068 to statistically significantly indicate the presence of SARS-CoV-2 RNA (to >2 sigmas of significance) from the Oropharyngeal swabs and Urine samples of patients that tested negative for measles despite morbilliform eruptions within Lombardy, Northern Italy, the other two studies that covers the same time period in Lombardy, two studies that investigated the presence of SARS-CoV-2 antibodies in patient samples from Lombardy indicate that the presence of SARS-CoV-2 specific antibodies within elderly patients that were being screened for lung cancer (that is, patients that have recently experienced respiratory problems), suggested that the discovery of SARS-COV-2 specific antibodies clustered temporally with the discovery of SARS-CoV-2 RNA within the prepandemic period in Italy—thus providing independent confirmation of a pre-pandemic wave of SARS-CoV-2 circulation within this location prior to the first official case of SARS-CoV-2 infection in Wuhan.
https://journals.sagepub.com/doi/full/10.1177/0300891620974755
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8778320/
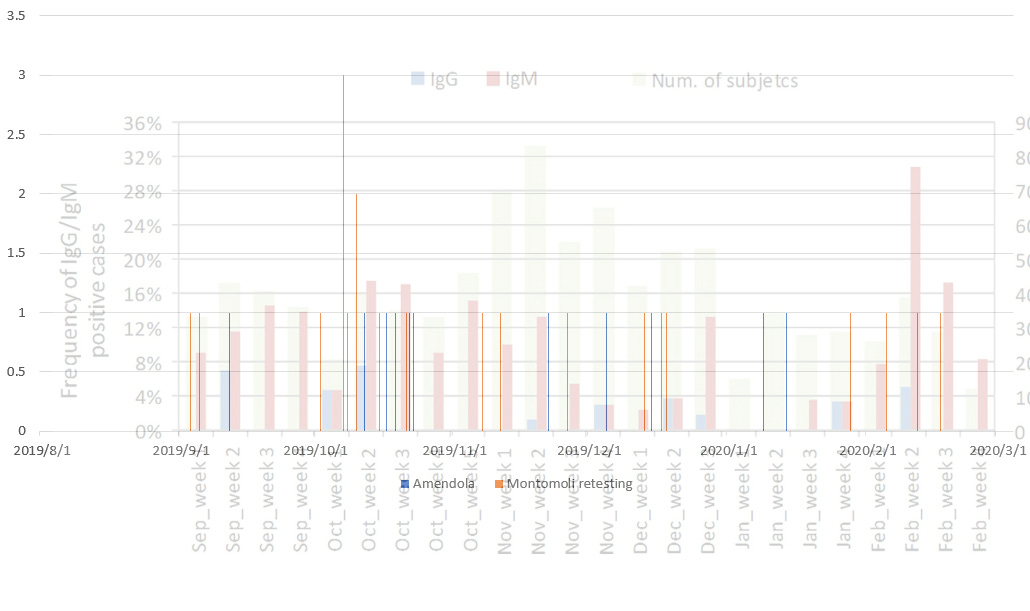
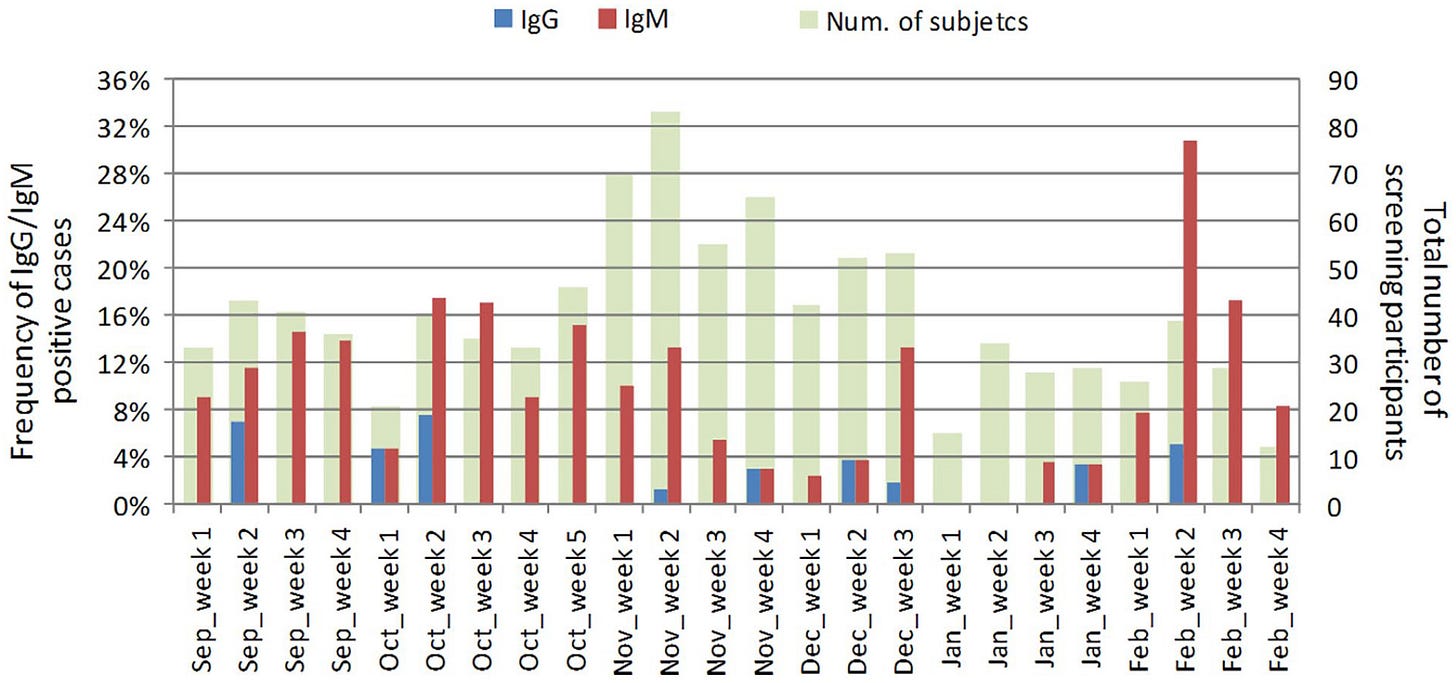
The SMILES cohort, which analyzed lung cancer screening samples for the presence of SARS-CoV-2 specific antibodies over the time period of September 2019-February 2020, and one of the first research articles documenting the discovery of the pre-pandemic circulation of SARS-CoV-2 before the first known case of SARS-CoV-2 infection at the Huanan seafood market in Wuhan, reported that IgG antibodies targeting the RBD protein of SARS-CoV-2 were found in the week 2 of September 2019, Week 1 and week 2 of October 2019, Week 2 and Week 4 of November 2019, Week 2 and Week 3 of December 2019, Week 4 of January 2020 and Week 2 of February 2020. As People are more likely to book tests associated with lung cancer screening at medical institutions, become aware of the program, get enrolled, and have their blood taken if they have recently experienced respiratory ailments, an enrichment of recent symptomatic respiratory infections within the initial blood draw (which the samples that would be used to retrospectively screen for SARS-CoV-2 antibodies were saved from) of the patients within this cohort is likely to be present, selectively enriching people with potential recent SARS-CoV-2 infections (the number of people with recent respiratory ailments) above the overall seroprevalence of the general population (the entire population size of the trial location).
In order to validate the result of the initial SMILES cohort, validation tests were performed in two independent laboratories that received a total of 29 pairs of SMILES cohort and date-in-year of collection, sex, age, and smoking habits-matched samples collected between 14 July 2018 and 23 February 2019. Samples that were positive for at least one of the tests were found beginning on 3 September 2019.
By plotting the date of collection for the positive samples (RNA PCR (Amendola et.al.), RBD-specific in-house IgG, and Positive commercial tests) together on one date-matched chart, it is found that the positive SARS-CoV-2 RNA results from the "young" samples (age <20 y, given measles screening is conducted mostly on children) coincided in time with the detection of IgG antibodies from aged lung cancer patients in the initial Montomoli et. al paper, and clustered detection of antibodies within the 2022 retesting effort.
This is expected as children are most likely infected by adults during the transmission of SARS-CoV-2, with or without respiratory symptoms.
The positive SARS-CoV-2 RNA results from the "non-young" samples (from mid-December 2019 to early-January 2020, age >20 y as the evolving virus begins to manifest dermatological symptoms in adults) were found to be ~2 weeks before the detection of IgG within the initial Montomoli et. al. SMILES paper.
This is also expected as adult infections take on average ~14 DPI to begin generating IgG.
Although the period of study was not explicitly given, the first detection of SARS-CoV-2 RNA and antigens within a sample from an adult with Morbiliform eruptions, the dermatosis sample reported by Gianotti et. al. on 10/11/2019, incidentally, also lands ~2 weeks before the detection of IgG on "Week 2 of November" by Montomoli et. al.
Amandola et. al (using PCR methods for detection of SARS-CoV-2 nucleic acids), Montomoli et. al (testing for SARS-CoV-2 RBD-specific antibodies), and Gianotti et. al. (testing for SARS-CoV-2 RNA and antigens using RNA-FISH and Immunohistochemistry) used entirely different (and orthogonal) technologies. Despite none of these efforts sharing even a single author with one another, the timeline (temporal signal and clustering of positive detections) generated from these independent tests matched each other closely. This indicates that an outbreak of infections that tests positive for SARS-CoV-2 RNA by in-situ hybridization, returns SARS-CoV-2 sequences when sequenced, leaves behind SARS-CoV-2 antigens and generates antibodies reactive to SARS-CoV-2 have happened within Northern Italy over the course of September-December 2019, prior to the first case of SARS-CoV-2 infection within the Huanan seafood market and before the officially recognized beginning of the SARS-CoV-2 pandemic.
Although the prevalence, severity, and extent of respiratory symptoms during this outbreak are largely unknown, this outbreak is clearly capable of generating measles-like morbilliform eruptions and is capable of causing respiratory infections (as SARS-CoV-2 RNA are found within Oropharyngeal swab samples taken from this period), and evolutionary changes within the virus backbone leading to changes in the pathophysiology and tropism of the virus, may have happened in this period of time.





There was a push to vaccinate the elderly in Italy the fall of 2019 with flu vaccines. I can’t help but wonder if they tested mRNA vaccines or possibly some experimental LAV within those lots, and if so, could that be related in any way to the evolution of SARSCOV2.
Covid-19, a virus so severe and deadly it circulated worldwide undetected for 3 months.